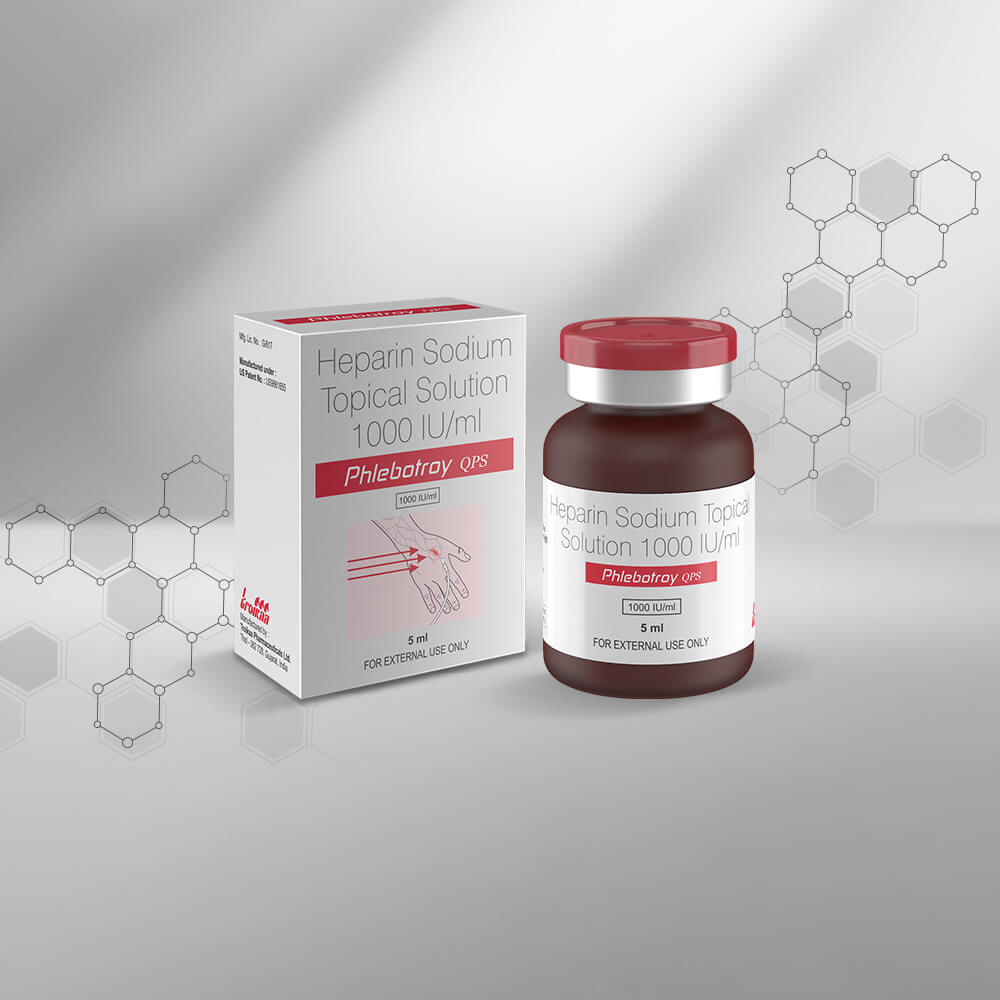
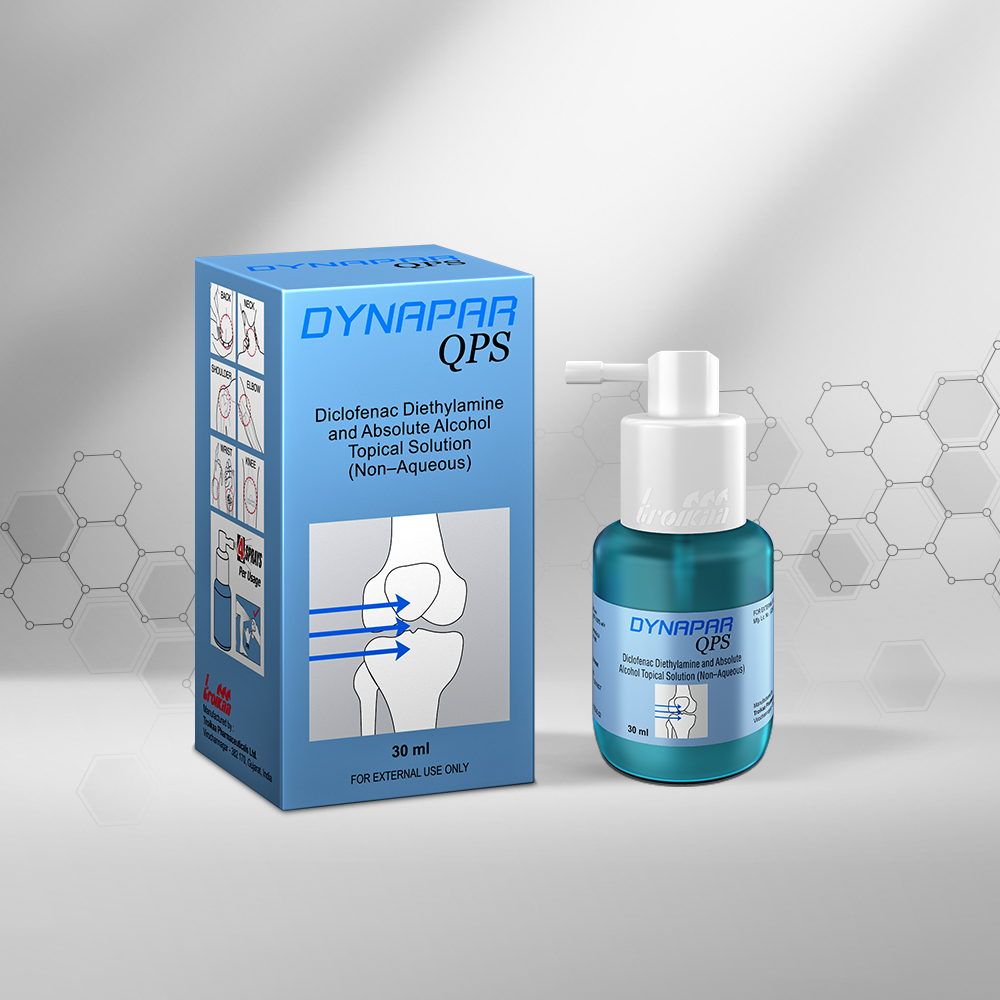
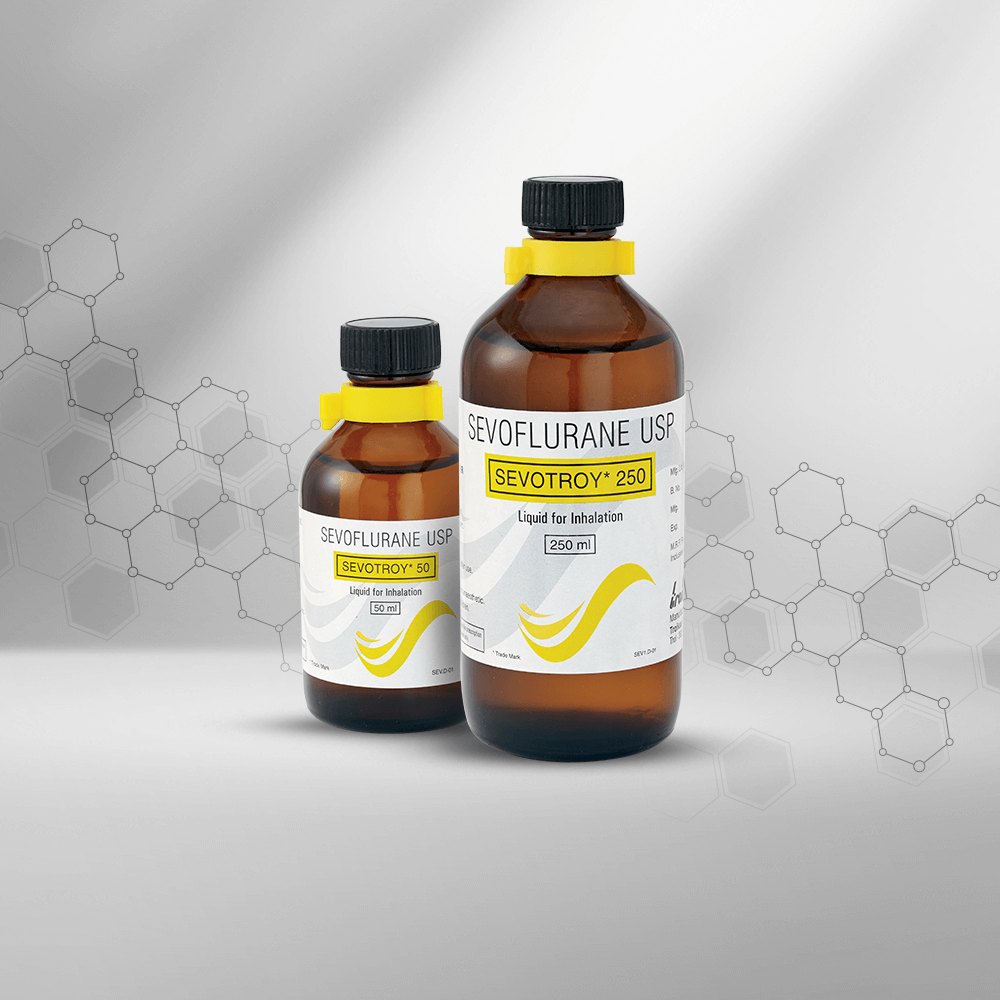
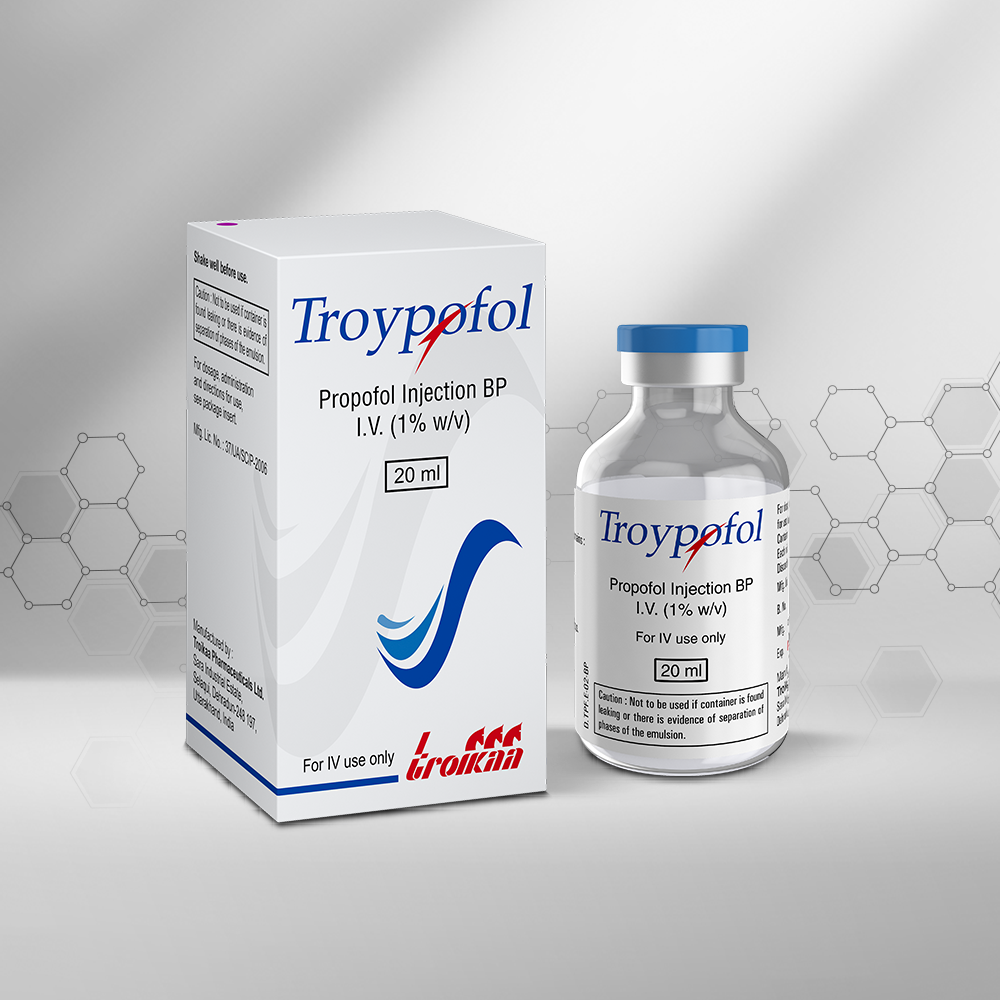
We are in the business of providing a healing touch to more than 120 countries worldwide. With our multicultural workforce comprising of more than 25 nationalities, we have innovative products that offer therapeutic solutions to regions like Asia, Africa, Latin America and CIS (Commonwealth of Independent States). Our unique NDDS product basket has helped us emerge as an eminent player in therapeutic segments like Pain Management, Critical Care, Anesthesiology and Cardiology. Our strong Regulatory Affairs Department creates documents that comply with all the regulatory norms applicable for various countries. We also have a global Sales Team, a Marketing Team and a Business Development Team that collectively spearheads our International business across the globe. Our success story is backed by our foundation of having three state-of-the-art manufacturing facilities approved by WHO – GMP, PIC/S(The Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme), ANVISA – Brazil, INVIMA Colombia and various other regulatory agencies. We are all set to get our manufacturing plants accredited with EU – GMP and USFDA.

Changing lives
across 120 countries